Alzheimer’s Research & Therapy volume 15, Article number: 173 (2023)
Abstract
Background
It is unknown if fluid biomarkers reflective of brain pathologies are useful in detecting and following a neurodegenerative process in individuals exposed to repetitive head impacts. This study explores the relationship between blood biomarkers and longitudinal change in cognitive function and regional brain volumes in a cohort of professional fighters.
Methods
Participants are drawn from a convenience sample of active and retired professional boxers and Mixed Martial Arts fighters and a control group with no prior exposure to head impacts. 3 T MRI brain imaging, plasma samples, and computerized cognitive testing were obtained at baseline and, for a subset, annually. MRI regional volumes were extracted, along with plasma levels of neurofilament light chain (NfL), glial fibrillary acidic protein (GFAP), p-tau231, and N-terminal tau (NTA). Statistical analyses were performed to assess the relationship between plasma levels and regional brain volumes and cognitive performance at baseline and longitudinally.
Results
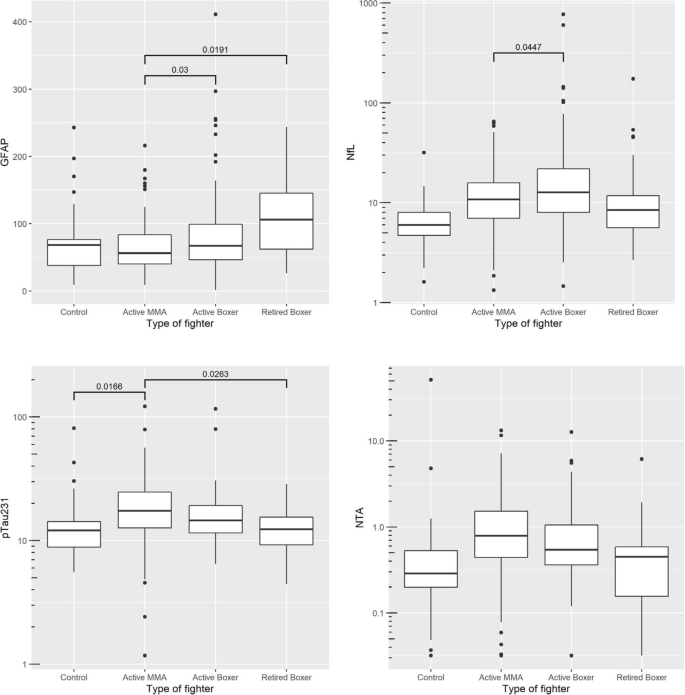
One hundred forty active boxers (mean age: 31 with standard deviation (SD) of 8), 211 active MMA (mean age of 30 with SD of 5), 69 retired boxers (mean age 49 with SD of 9), and 52 control participants (mean age 36 with SD of 12) were included in the analyses. Baseline GFAP levels were highest in the retired boxers (retired boxers v. active MMA: p = 0.0191), whereas active boxers had higher levels of NfL (active boxers v. MMA: p = 0.047). GFAP showed an increase longitudinally in retired boxers that was associated with decreasing volumes of multiple cortical and subcortical structures (e.g., hippocampus: B = − 1.25, 95% CI, − 1.65 to − 0.85) and increase in lateral ventricle size (B = 1.75, 95% CI, 1.46 to 2.04). Furthermore, performance on cognitive domains including memory, processing speed, psychomotor speed, and reaction time declined over time with increasing GFAP (e.g., processing speed: B = − 0.04, 95% CI, − 0.07 to − 0.02; reaction time: B = 0.52, 95% CI, 0.28 to 0.76). Among active fighters, increasing levels of GFAP were correlated with lower thalamic (B = − 1.42, 95% CI, − 2.34 to -0.49) and corpus callosum volumes, along with worsening scores on psychomotor speed (B = 0.14, 95% CI, 0.01 to 0.27).
Conclusion
Longitudinal plasma GFAP levels may have a role in identifying individuals exposed to repetitive head impacts who are at risk of showing progressive regional atrophy and cognitive decline.
Background
Extensive exposure to repetitive head impacts (RHI) increases the risk of long-term neurological impairment including chronic traumatic encephalopathy (CTE) [1]. However, not everyone exposed to RHI will experience neurological decline and among those who do, the onset of symptoms may be many years or decades after exposures [2]. Because of this, the ability to predict or track changes occurring in the brain in either those still, or previously, exposed to RHI has numerous implications both on a personal and research basis. To this end, there is a great interest in identifying biomarkers that could be used to detect the development of a neurodegenerative process and/or follow progression over time [3, 4].
Among biomarkers under investigation, most are either imaging or fluid (blood or CSF) based. As has been seen in the field of Alzheimer’s disease, blood biomarkers have the potential to be used to screen or support a clinical diagnosis or become an outcome measure in clinical therapeutic trials [5, 6]. With the availability of sensitive blood-based assays, a number of candidates have been studied in traumatic brain injury including neurofilament light chain (NfL), glial fibrillary astrocytic protein (GFAP), and various species of tau [7, 8]. However, much of the prior longitudinal research with these measures has had relatively short follow up, limited outcome measures, or studied groups exposed to single traumatic brain injuries of varying severities [9,10,11].
The Professional Athletes Brain Health Study is a longitudinal cohort study of both active and retired professional fighters. Utilizing this well characterized cohort of individuals exposed to RHI and followed over time, we chose four plasma biomarkers to examine: GFAP (a marker of astrocytic injury or activation), NfL (a marker of neuroaxonal injury and degeneration), p-tau231 (a marker of tau phosphorylation), or N-terminal tau ([NTA tau] a novel plasma biomarker specific for AD pathology). The primary aims of the study were to determine (1) whether baseline biomarker levels were correlated with cognitive performance or MRI regional volume in individuals exposed to RHI, (2) whether baseline biomarker levels predict subsequent change over time in cognition or MRI regional volumes, and (3) does longitudinal trajectory of these markers correlate with change over time in the outcomes?
Methods
Cohort
The Professional Athletes Brain Health study (PABHS) is composed of active and retired professional fighters (boxers and mixed martial artists), along with controls. Active fighters were required to have at least 1 professional fight within 2 years of enrollment and be training with the intent to compete. Retired fighters were included if they had been boxers or mixed martial artists, had a minimum of 10 professional fights, had no sanctioned fights for at least 2 years, and did not intend to return to competition. Control subjects were recruited from outreach efforts in the community and could not have any prior history of neurological disorders, head trauma, military service, or participation at a high school level or higher in a combat sport or a sport in which head trauma can be anticipated to occur, such as football, wrestling, hockey, rugby, soccer, or rodeo. Enrollment in the PABHS began in 2011 and has been continuous since then. Each participant is seen on an annual basis and, for active fighters, not sooner than 45 days from a sanctioned fight to reduce the potential acute effects of head impacts sustained in competition. Because of a variety of reasons (training and competition schedule, travel issues, other obligations), participants who missed a study visit were allowed to remain in the study with the next study visit conducted as soon as they were available. We consider the “baseline” blood levels as the ones that were drawn at the first study visit. Data for this study were collected between 2011 and 2018.
Procedures
At each visit, blood sampling is obtained, along with a battery of other tests including MRI brain imaging, computerized cognitive testing, and exposure information. The PABHS was approved by the Cleveland Clinic Institutional Review Board, and written informed consent was obtained from all participants. Methods of recruitment and study procedures have been described previously [12].
Cognitive function was assessed by a computer-based battery consisting of four subtests of the CNS Vital Signs (CNS Vital Signs, North Carolina) including verbal memory, symbol digit coding, Stoop, and a finger tapping test. CNS Vital Signs offers robust and reliable measurements of cognition which are computerized but are supervised by a technician [13]. Results from these tests are used to make up scores in various clinical domains: verbal memory, processing speed, psychomotor speed, and reaction time. Raw scores were used in the analyses.
A high-resolution T1-weighted anatomical MRI was obtained on a 3 T MRI scanner (Siemens Verio from April 2011 through October 2015 and Siemens Skyra from December 2016 to the present) with a 32-channel head coil to acquire structural three-dimensional T1-weighted magnetization prepared rapid acquisition gradient echo images (repetition time msec/echo time msec, 2300/2.98; resolution, 1 X 1 X1.2 mm3. Volumes of the hippocampus, amygdala, superior temporal, various frontal regions, anterior cingulate, and total gray matter and subcortical grey matter including thalamus, caudate, and putamen, along with corpus callosum and total white matter volume, were calculated using the automated full brain segmentation process in the Freesurfer 6.0 software. These regions have been shown in pathological series and our prior work to be affected in those with extensive RHI [14, 15]. The volumes of each structure were measured in both hemispheres separately and an average volume calculated for structures that have bilateral representation. The regional volumes were adjusted for total intracranial volume (TIV) by adding TIV as a covariate. A quality control step was performed using the FreeSurfer’s quality analysis tools (https://surfer.nmr.mgh.harvard.edu/fswiki/QATools) to guarantee only data with high-quality cortical reconstruction from FreeSurfer were included in the analyses.
The blood samples were collected in EDTA tubes and centrifuged at 3200 rpm for 10 min to separate plasma from blood cells. The supernatant was aliquoted in 2 ml portions that were immediately frozen and stored at – 80° pending analysis. For all measured biomarkers (commercially available or in-house developed), plasma samples were allowed to thaw at room temperature for 45 min, after which they were vortexed (at 400 rpms for 30 s) and centrifuged (4000 g for 10 min). Internal quality controls (iQC) were included on each plate before and after the analyzed samples to determine inter- and intra-assay variability (intra- and inter-assay variation was < 15% for all biomarkers). All blood biomarkers were measured using a Simoa HD-X platform (Quanterix, Billerica, MA, USA) at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. NfL and GFAP were measured using commercially available Simoa kits (NF-light™ #103,186 and GFAP #102,336, Quanterix, Billerica, MA, USA) following manufacturer specifications. In-house developed plasma p-tau231 and NTA tau are measured following published protocols [16, 17]. In brief, plasma p-tau231 assay is comprised by a mouse monoclonal antibody selective against phosphorylated tau at threonine 231 (ADx253, ADx Neuroscience) and a biotinylated mouse monoclonal antibody with epitope at aa 6–18 (Tau12, #806,501, Biolegend). Recombinant full-length Tau-441 phosphorylated in vitro by glycogen synthase kinase 3β (#T08-50FN, SignalChem) was used as the calibrator. The plasma NTA assay targets N-terminal tau fragments using two mouse monoclonal antibodies, with epitopes at aa 6–18 (Tau12, #806,501, Biolegend) and 194–198 (HT7, #MN1000, Thermo Scientific). Recombinant non-phosphorylated full-length Tau-441 (#T08-54N, SignalChem) was used as the calibrator.
Genotyping of apolipoprotein E (APOE) alleles was performed using real-time PCR restriction fragment length polymorphism analysis. Briefly, genomic DNA was collected from blood DNA extracted using Qiamp DNA blood maxi kit (Qiagen), and APOE genotyping was performed using Applied Biosystems TaqMan SNP Genotyping Assay.
STROBE reporting guideline was adhered to in preparing this manuscript.
Statistical analysis
The cohort was divided into four groups for analyses: active boxers, active MMA fighters, retired boxers, and controls. We chose to divide the active fighters by their fighting discipline because of prior findings from the PABHS that indicate active boxers show lower regional gray matter volumes and lower scores on cognitive tests after adjusting for number of professional fights and other factors compared to the active MMA fighters [15, 18]. We examined only retired boxers because we did not have enough retired MMA fighters in the cohort to analyze separately.
For the comparison of demographic data between the four groups in this study cohort, Kruskal–Wallis test was used for continuous outcomes (e.g., age, number of fights), and the chi-squared test was used for categorical outcomes (e.g., race). For continuous outcomes, we reported the interquartile range (IQR) values with the mean value. For APOE ε4 positivity, Fisher’s exact test was used for comparing the four groups [19].
We used linear regression models to compare the baseline blood biomarkers (GFAP, NfL, p-tau231, NTA) between the four groups after controlling for age, gender, race, education years, and number of fights (Fig. 1). Race was included as a covariate because of recent reports from the Alzheimer’s disease literature that suggest some fluid biomarkers may vary by race [20, 21]. In the PABHS cohort, race was determined by self-report and included White, African American, Asian, Pacific Islander, and American Indian/Native Alaskan. Those who did not designate a race were placed into the category of other. We chose to adjust for number of fights as previous findings from the PABHS indicate that this exposure measure itself predicts both cognitive and MRI volumetric outcomes [22]. In Fig. 1, the reported p-values are the ones after the multiple testing correction by using the Tukey’s approach.
For the association between blood biomarkers at baseline and cognitive performance or MRI regional volume at baseline, linear regression models were performed with the covariates: age, gender, race, education years, and number of fights. Though we would have preferred to compare our fighter groups to the controls, we felt we did not have enough controls to match either the younger active fighter groups or the older retired boxer group. Furthermore, plasma levels of all the biomarkers we studied are known to be influenced by age. Thus, we performed within group analysis. When MRI regional volumes are the outcome of interest, another two covariates were added to the statistical model: scanner type and the total intracranial volume (TIV). These two covariates were also added in the following statistical models for repeated MRI regional volumes.
Participants were included in longitudinal analyses if they had two or more visits including blood biomarker, MRI, and cognitive data. Linear mixed effect models were used to assess the relationship between the longitudinal cognitive performance or MRI regional volume and each longitudinal blood biomarker data. As above, each group was analyzed separately. The outcomes are MRI regional volumes or cognitive measures. The fixed effects are blood biomarker, group, and their interaction, age, gender, race, education years, and number of fights. The fixed effect group is a categorical variable with 4 separate arms. The other two covariates, scanner type and the total intracranial volume, were added in the models for repeated MRI regional volumes. The correlation for outcome from the same participant is assumed to be the compound symmetry structure. We also ran linear mixed models to evaluate the relationship between baseline blood biomarker level and the longitudinal cognitive performance or MRI regional volume. The same fixed effects were included in the model.
We checked the statistical model assumptions by virtually inspecting the following plots: residual VS fitted value plot, Q-Q plot. The statistical software SAS was used in the analyses, and software R was used for some plots. All the tests are two-sided with the significance level of 0.05.
Results
The study cohort was primarily made up of active boxers (n = 140) and MMA (n = 211) fighters with a smaller number of retired boxers (n = 69) and controls (n = 52). Demographic and clinical data are presented in Table 1. We also ran post-hoc tests using the Bonferroni method for multiple comparisons. There was a group difference in age except between the active boxers and the active MMA. Education years and number fights were different between fighter groups in the post-hoc comparisons.
The number of participants in the longitudinal analyses included 52 active boxers, 55 retired boxers, 103 active MMA, and 27 controls. This longitudinal subgroup had average years of follow up ranging from 2.73 (SD = 1.52, IQR 1.5–4) in the retired boxers to 3.35 (SD = 1.44, IQR 2–4) for active boxers, 3.09 (SD = 1.57, IQR 2–4) for active MMA, and 2.11 (SD 1.01, IQR 1–3) for controls.
Baseline levels between groups
Baseline GFAP levels were significantly increased in both retired boxers (mean = 108, SD = 56) and active boxers (mean = 81, SD = 59) compared with active MMA fighters (mean = 65, SD = 35) [retired boxer v. active MMA: p = 0.019, active boxer v. active MMA: p = 0.03]. NfL increased in active boxers (mean = 30.28, SD = 83.85) as compared to the active MMA (mean = 13.85, SD = 10.95), with p = 0.0447. Plasma p-tau231 was increased in active MMA fighters compared with retired boxers (p = 0.026) and control (p = 0.0166). Plasma NTA baseline levels were not significantly different across groups (Fig. 1).
After removing two outliers with NfL values above 500, the mean NfL difference between active MMA and active boxers was reduced, but the standard error of the mean difference was reduced even more due to smaller variance. For that reason, the adjusted p-value for comparing active MMA and active boxers was 0.006. In addition, the difference between active boxers and controls became statistically significant with the adjusted p-value of 0.0002.
Baseline levels and volumetric/cognitive outcomes
Cross-sectional analysis within groups revealed relationships between higher levels of GFAP and lower volumes in various gray and white matter regions and higher ventricular volumes primarily in the retired boxers (Table 2). The structures effect most in the retired boxers included thalamus (B = − 4.12, 95% CI, − 6.44 to − 1.81) hippocampus (B = − 1.90, 95% CI, − 3.46 to − 0.34), inferior lateral ventricle (B = 1.46, 95% CI, 0.15 to 2.76), cerebral white matter (B = − 40.72, 95% CI, − 70.4 to − 11.1), and total gray matter (B = − 7.92, 95% CI, − 11.91 to − 3.92). In addition, for retired boxers, lower scores on processing speed (B = − 0.25, 95% CI, − 0.38 to − 0.11) were associated with higher levels of GFAP (Additional file 1: Fig. S3). Among the active boxers, higher GFAP levels correlated with lower volumes in the thalamus and larger volumes of the lateral ventricles. Within-group assessment of NfL showed that higher baseline levels were associated with lower volumes of the thalamus (B = − 1.68, 95% CI, − 2.77 to − 0.61), hippocampus (B = − 0.84, 95% CI, − 1.56 to − 0.112), anterior cingulate (B = − 0.34, 95% CI, − 0.61 to − 0.06), and subcortical gray matter (B = − 7.90, 95% CI, − 14.3 to − 1.47) in the active boxers. On the other hand, no consistent relationships were seen with the volumetric measures and levels of ptau 231 and NTA in any of the groups.
Baseline level of GFAP correlated with longitudinal rate of change in both MRI volume and cognitive measures primarily within the retired boxer group. For every unit increase in GFAP at baseline, there was a greater yearly rate of decline in the thalamus (B = − 4.64, 95% CI, − 6.74 to − 2.54), hippocampus (B = − 2.52, 95% CI, − 3.86 to − 1.18), amygdala (B = − 0.67, 95% CI, − 1.33 to − 0.01), and increase in the inferior lateral ventricle (B = 2.59, 95% CI, 1.43 to 3.76). There was also a decline in processing speed (B = − 381, 95% CI, − 552 to − 210) and reaction time (B = 0.77, 95% CI, 0.31 to 1.23). Baseline measures of the other 3 analytes did not correlate with longitudinal outcomes.
Longitudinal change and volumetric/cognitive outcomes
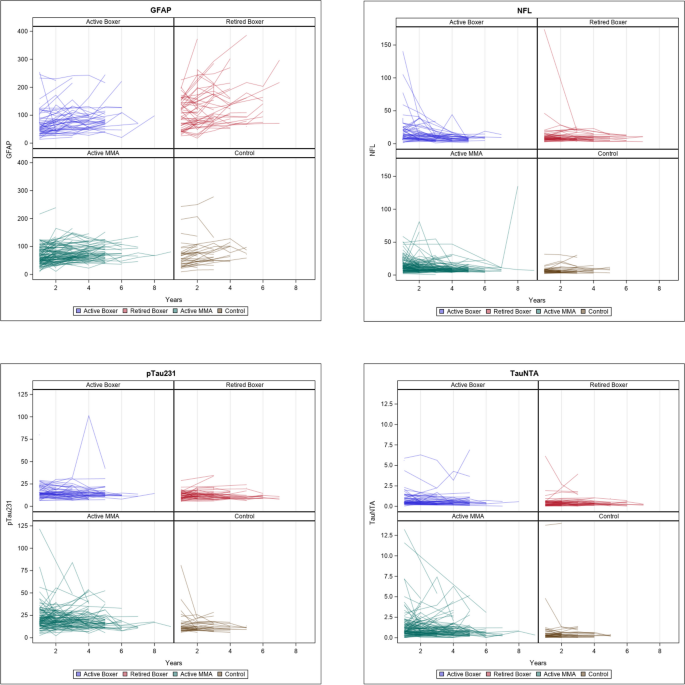
In Fig. 2, we show the longitudinal change of each blood biomarker. Longitudinal repeated-measure models were used to assess the change over time after controlling for the aforementioned covariates. GFAP is the only analyte showing an increase longitudinally. We then further analyzed the longitudinal change of GFAP with the longitudinal change of MRI volumes and cognitive measures in Table 3.
Longitudinal increase in GFAP in retired fighters was associated with decreasing volume of multiple cortical and subcortical structures (e.g., hippocampus (left): B = − 0.28, 95% CI, − 0.49 to − 0.0.08) and increase in lateral ventricle size (B = 2.19, 95% CI, 1.53 to 2.85) as reported in Table 3. Furthermore, performance on a variety of cognitive domains including memory, psychomotor speed, and reaction time declined over time with increasing GFAP (e.g., psychomotor speed: B = − 0.02, 95% CI, − 0.038 to − 0.002; reaction time: B = 0.20, 95% CI, 0.097 to 0.303) (see Additional file 2: Fig. S4). In the active boxer group and the active MMA group, a relationship was seen between increasing levels of GFAP and increase in lateral ventricle size.
Discussion
In pursuit of biomarkers that may be applicable in detecting progressive neurological change in those exposed to repetitive head impacts, this study evaluated several blood-based measures in a cohort of both active and retired professional fighters, a subset of which had longitudinal imaging and cognitive assessments. In answer to the aims of this study, we found that (1) among the analytes tested, higher levels of GFAP were inversely correlated with regional volumes and cognitive performance in retired boxers. On the other hand, NfL levels were inversely associated with volume measurements at baseline in active boxers. (2) Higher levels of baseline GFAP level were associated with increasing rate of cognitive and MRI volume decline in retired boxers, and (3) increasing levels of GFAP over time were inversely related to rate of decline in retired boxers. The N-terminal tau assay (NTA) and p-tau231 did not seem to have any clear relationships to the outcome measures.
How might the findings of this study translate to clinical or research use, either to screen for (or determine risk of) a neurodegenerative process such as CTE or be employed as a biomarker in clinical trials? To begin with, cross-sectional measurements of any of these biomarkers may not have utility as a diagnostic measure for a condition such as CTE. There was a clear overlap in plasma levels of all biomarkers between our active and retired fighters and control subjects who have not been exposed to RHI. Previous studies of all the blood biomarkers we tested have reported as high or higher absolute levels in other neurodegenerative disease states [23]. What makes it difficult to compare absolute values of these blood constituents between studies are the differences in technical factors such as how the samples are handled and processed and the platforms used for the measurements. In addition, elevated levels of GFAP and NfL (among a number of other plasma biomarkers) have been described following acute exposure to TBI and RHI [4].
On the other hand, following plasma GFAP levels over time may help identify those previously exposed to RHI who are developing a neurodegenerative process such as, but not limited to, CTE. GFAP is an intermediate filament protein that is predominately expressed in astroglial cells and thought to be a marker of astrocyte remodeling and reactivity [24]. Prior studies have shown that GFAP increases with age and may be a marker of Alzheimer’s disease [25]. However, elevations have been reported in other neurodegenerative conditions and GFAP has also demonstrated a biphasic release in blood after acute severe TBI, with initial increase, followed by decreasing levels over the first 6 months and then subsequent increase [7]. In our study, the increasing levels of GFAP in the retired boxers may reflect underlying neuroinflammation and/or astrogliosis manifest by regional volume loss and associated clinical finding of lower performance on cognitive measures. However, in the absence of pathological confirmation, there is no way to truly know what type of pathophysiology the plasma GFAP represents. Furthermore, our findings need to be replicated in other cohorts exposed to RHI.
The other biomarkers we studied may have different applications. Levels of NfL were higher at baseline in active boxers than active MMA fighters and were associated with lower thalamic, hippocampal, and white matter volumes in that group. NfL, an axonal component found primarily in large caliber myelinated subcortical fibers, is one of the more widely studied fluid biomarkers and is thought to be released with axonal injury [26, 27]. Elevated levels of NfL can be seen after acute TBI, with some studies indicating a slow return to baseline extending over the years [7]. Higher levels have also been reported in several neurological diseases such as amyotrophic lateral sclerosis, progressive supranuclear palsy, and Alzheimer’s disease (AD) [28,29,30]. We propose that the increased levels of NfL in the active boxers may reflect axonal injury from the numerous blows to the head that boxers generally sustain in training and competition; the lower regional volumes perhaps represent the subsequent effects of Wallerian degeneration and neuronal atrophy. The absence of elevated NfL in the retired boxers at baseline and longitudinally differs from what has been described in other neurodegenerative conditions such as AD; though the reason is unclear, it may be that the underlying pathological process associated with RHI in this cohort is more indolent.
The characteristic pathological feature in CTE is the presence of perivascular p-tau deposits at the depths of sulci [10]. Consequently, there has been an interest in evaluating the performance of p-tau measures in CSF or blood as a diagnostic for CTE. Prior work has reported that higher levels of p-tau can be seen soon after TBI which likely represents acute injury [6].
However, the situation is more complicated in trying to use p-tau measures to detect CTE. Tau can be phosphorylated at a variety of sites and there is no agreement on those that are specific or associated with CTE. A recent study that evaluated two p-tau species (p-tau 181, p-tau 217) in a group that included a small number of postmortem verified CTE cases found that these markers were specific for Alzheimer’s disease and not in vivo biomarkers of CTE tau [31].
Among tau phosphorylation sites, those that have been reported with CTE include p-tau 175, 202, 231, and 396 [32, 33]. We chose to study p-tau 231 because it also has been suggested to be specific to the pathology that underpins Alzheimer’s disease, even at the preclinical phase [34]. Similarly, increased blood levels of NTA, which measures non-phosphorylated N-terminal tau species, have been reported to be specific for Alzheimer’ disease and increased in symptomatic patients but little data exists on its performance in other neurological conditions [35]. We did not find that levels of p-tau231 or NTA were related to any of our outcome measures. Our findings suggest that these tau biomarkers are not a marker of a neurodegenerative process related to prior exposure to RHI; the generally flat trajectory of both p-tau 231 and NTA tau over time in the retired boxers also may support the notion that Alzheimer’s disease was not the underlying condition causing the progression seen in MRI volumetrics and cognition in this group.
While this study benefits from having longitudinal biomarker levels and corresponding imaging and cognitive measurements in a large population of individuals with exposure to RHI, there are limitations to discuss. To begin with, because this is not a clinicopathological study, it is impossible to know what process is driving the regional volume changes that were seen in association with some of the fluid biomarkers. In addition, the PABHS does not represent a random sample of professional fighters, and the longitudinal nature of the study raises the possibility that those who participate and are retained in the study may differ in some way from their peers. Another issue regarding the study cohort is our inability to accurately know how much exposure to RHI any individual had. Previous work from the PABHS has indicated that the number of professional fights is a reasonably good surrogate of exposure and is what we employed in adjusting our analyses [22]. There were several differences between fighter groups and controls that limited direct comparison between them and led us to within group analyses. Our control group had the fewest participants and also differed in age than the retired fighters (though there was overlap), being generally younger. Thus, we did not have sufficient numbers of older control participants to compare with the retired fighters. The control group also had a higher percentage of white participants than the fighter groups. The groups also differed in the number of longitudinal plasma samples. The change in MRI scanner during the study period was addressed by adjusting for scanner type in the analyses but could possibly introduce some variability in the regional volume measurements. Plasma biomarker levels could potentially be influenced by BMI which we did not have on all the participants. Finally, there may be technical factors that influence the longitudinal results; many of these samples did undergo multiple freeze/thaw cycles. However, earlier studies have shown that NfL, GFAP, and tau markers are stable even with repetitive freeze/thaw cycles [36].
To conclude, employed as a longitudinal measure, plasma GFAP levels may have a role in identifying individuals who are at increased likelihood of showing progressive regional atrophy and cognitive decline and perhaps could be an outcome measure in clinical trials. On the other hand, NfL measurements seem to be more applicable in those actively exposed to RHI in reflecting neural injury. Further longitudinal studies over greater amounts of time and with different cohorts exposed to RHI are needed to verify our findings.
Availability of data and materials
The datasets used for the current study are available from the corresponding author on reasonable request.
References
-
Alosco ML, Stern RA. The long-term consequences of repetitive head impacts: chronic traumatic encephalopathy. Handbook Clin Neurol. 2019;167:337–55.
-
Asken BM, Sullan MJ, DeKosky ST, Jaffee MS, Bauer RM. Research gaps and controversies in chronic traumatic encephalopathy: a review. JAMA Neurol. 2017;74:1255–62.
-
Ehrenberg AJ, Khatun A, Coomans E, et al. Relevance of biomarkers across different neurodegenerative disease. Alzheimers REs Ther. 2020;12:56.
-
Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12:563–74.
-
Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022;21(8):726–34.
-
Zetterberg H, Schott JM. Blood biomarkers for Alzheimer’s disease and related disorders. Acta Neurol Scand. 2022;146(1):51–5.
-
Shahim P, Politis A, van der Merwe A, et al. Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology. 2020;9:e623-636.
-
Rubenstein R, Chang B, Yue J, et al. Comparing plasma tau, total tau, and phospho tau-total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol. 2017;74:1063–72.
-
Hier DB, Obafemi-Ajayi T, Thimgan MS, et al. Blood biomarkers for mild traumatic brain injury: a selective review of unresolved issues. Biomark Res. 2021;9(1):70.
-
Posti JP, Tenovuo O. Blood-based biomarkers and traumatic brain injury-a clinical perspective. Acta Neurol Scand. 2022;146(4):389–99.
-
McDonald SJ, Shultz SR, Agoston DV. The known unknowns: an overview of the state of blood-based protein biomarkers of mild traumatic brain injury. J Neurotrauma. 2021;38(19):2652–26666.
-
Bernick C, Banks S, Phillips M, et al. Professional Fighters Brain Health Study: rationale and methods. Am J Epidemiol. 2013;15:280–6.
-
Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery. CNS vital signs Arch Clin Neuropsychol. 2006;21:623–43.
-
McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–64.
-
Bernick C, Shan G, Zetterberg H, et al. Longitudinal change in regional brain volumes with exposure to repetitive head impacts. Neurology. 2020;94:e232–40.
-
Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021;141:709–24.
-
Snellman A, Lantero-Rodriquez J, Emersic A, et al. N-terminal and mid region tau fragments as fluid biomarkers in neurological diseases. Brain. 2022;145:2834–48.
-
Bernick C, Banks SJ, Shin W, et al. Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: the Professional Fighters Brain Health Study. Br J Sports Med. 2015;9:1007–11.
-
Shan G, Gerstenberger S. Fisher’s exact approach for post hoc analysis of a chi-squared test. PLoS ONE. 2017;12(12): e0188709.
-
Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9:88.
-
Hajjar I, Yang Z, Okafor M, et al. Association of plasma and cerebrospinal fluid Alzheimer Disease biomarkers with race and the role of genetic ancestry, vascular comorbidities and neighborhood factors. JAMA Netrw Open. 2022;5: e2235068.
-
Bernick C, Banks S. What boxing tells us about repetitive head trauma and the brain. Alzheimers Res Ther. 2013;5(3):23. https://doi.org/10.1186/alzrt177Benedet. A, Mila-Aloma M, Vrillon A, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer Disease continuum. JAMA Neurol.2021; 78(12): 1–13.
-
Moscoso A, Grothe M, Ashton N, et al. Longitudinal associations of blood phosphorylated Tau 181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78(4):1–12.
-
Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–35.
-
Katisko K, Cajanus A, Huber N, et al. Gfap as a biomarker in frontotemporal dementia and primary psychiatric disorders: diagnostic and prognostic performance. J Neurol Neurosurg Psychiatry. 2021;92:1305–12.
-
Bernick C, Zetterberg H, Shan G, et al. Longitudinal performance of plasma neurofilament light and tau in professional fighters: the Professional Fighters Brain Health Study. J Neurotrauma. 2018;35:2351–6.
-
Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88:1788–94.
-
Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. 2016;3:216–25.
-
Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–57.
-
Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer’s disease. JAMA Neurol. 2017;74:557–66.
-
Asken B, Tanner J, VandeVrede L, et al. Plasma p-tau 181 and p-tau 217 in patients with traumatic encephalopathy syndrome with and without evidence of Alzheimer disease pathology. Neurology. 2022;99:e594–604.
-
Stathas S, Alvarez V, Xia W, et al. Tau phosphorylation sites serine 202 and serine 396 are differently altered in chronic traumatic encephalopathy and Alzheimer’s disease. Alzheimer’s Dement. 2022;18:1511–22.
-
Moszczynski A, Strong W, Xu K, et al. Pathologic Thr175 tau phosphorylation in CTE and CTE with ALS. Neurology. 2018;90:e380–7.
-
Suarez-Calvert M, Karikari T, Ashton N, et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in ABeta pathology are detected. EMBO Mol Med. 2020;12: e12921.
-
Snellman A, Lantero-Rodriquez J, Emersic A, et al. N-terminal and mid-region tau fragments as fluid biomarkers in neurological diseases. Brain. 2022;145:2834–48.
-
Ashton NJ, Suarez-Calvert M, Karikari TK, et al. Effects of pre-analytical procedures on blood biomarkers for Alzheimer’s pathophysiology, glial activation, and neurodegeneration. Alzheimers Dement. 2021;13: e12168.
Acknowledgements
HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation program under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), and the UK Dementia Research Institute at UCL (UKDRI-1003).
KB is supported by the Swedish Research Council (#2017-00915 and #2022-00732), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the National Institute of Health (NIH), USA, (grant #1R01AG068398-01), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), and the Alzheimer’s Association 2022-2025 Grant (SG-23-1038904 QC).
Funding
Not applicable.
Ethics declarations
Ethics approval and consent to participate
The Professional Athletes Brain Health Study was approved by the Cleveland Clinic Institutional Review Board (#10–944), and written informed consent was obtained from all participants.
Consent for publications
Not applicable.
Competing interests
NJA has given lectures in symposia sponsored for Lily and Quanterix. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. CB has received research funding from UFC, Top Rank promotions, and Haymon Boxing.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S3.
Relationship between baseline GFAP (measured in pg/mL) and MRI volume measurements (in mm3) of the thalamus, hippocampus, and total gray matter, along with processing speed (computed from number of correct responses on Symbol Digit Coding test minus errors)
Additional file 2: Fig. S4.
Longitudinal measurement of reaction time ((Stroop Test Complex Reaction Time Correct + Stroop Reaction Time Correct)/2) and psychomotor speed [Finger Tap Test (FTT) Right Taps Average + FTT Left Taps Average + SDC Correct Responses) in active MMA fighters, active boxers, retired boxers, and control subjects)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.